These 20 Substances Have the Most Unusual Chemical Properties
In laboratories and nature alike, certain chemical substances behave in ways that seem to defy basic physics and chemistry. These remarkable compounds challenge scientific understanding and reveal the complexity of molecular interactions.
Here are substances with highly unusual chemical properties.
Water’s Quantum Weirdness

While seemingly ordinary, water exhibits bizarre chemical properties that make life possible. At 4 degrees Celsius, it becomes denser rather than less dense, allowing ice to float and lakes to support life beneath frozen surfaces.
Marine biologists note that without this strange property, Earth’s oceans would freeze from the bottom up.
Gallium’s Melting Touch

This silvery metal melts in human hands, transforming from solid to liquid at just above room temperature. Engineers harness this property in high-tech thermometers and computer cooling systems.
Laboratory demonstrations often feature gallium spoons that dissolve in hot tea, baffling first-year chemistry students.
Like Go2Tutors’s content? Follow us on MSN.
Sulfur Hexafluoride’s Heavy Voice
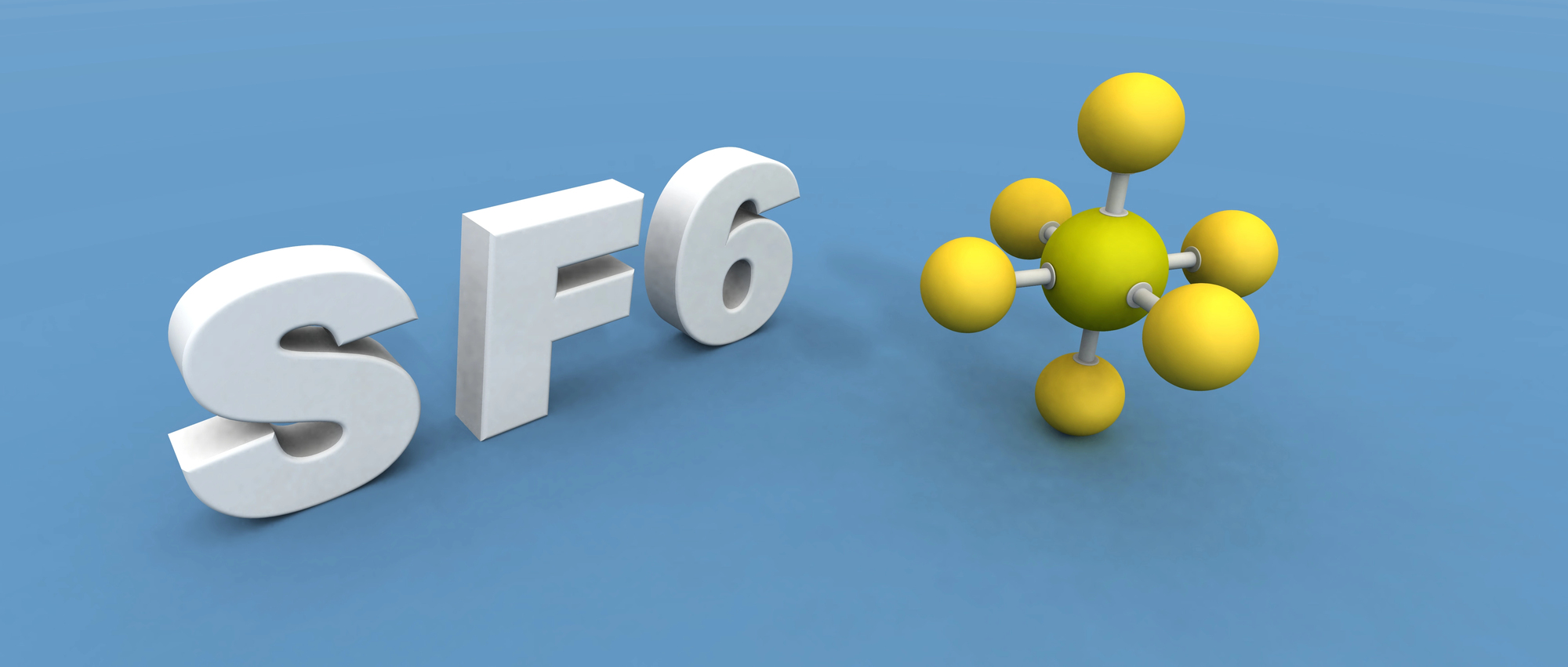
This gas has the opposite effect of helium on human voices, making them extraordinarily deep. Six times heavier than air, it sinks to the ground in transparent pools.
While useful in electrical insulation, physics professors often demonstrate its properties by floating aluminum boats on invisible lakes of gas.
Ferrofluids’ Magnetic Dance

These liquid magnets form hypnotic spikes when exposed to magnetic fields. Originally developed by NASA for rocket fuel control in zero gravity, ferrofluids now find applications in everything from computer hard drives to advanced car suspensions.
Artists use them to create otherworldly sculptures that seem to defy gravity.
Aerogel’s Ghost Matter
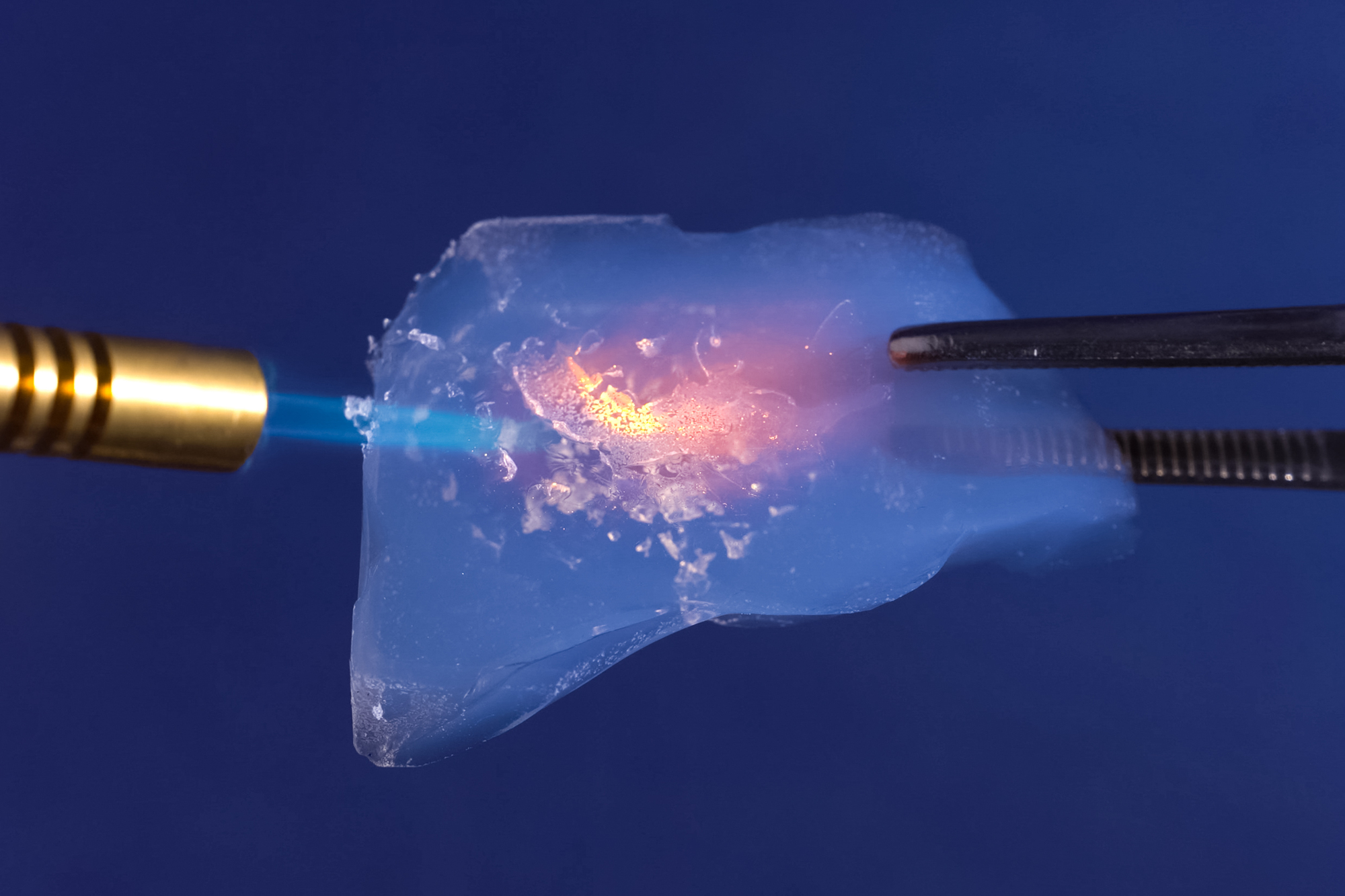
Known as “frozen smoke,” aerogel is the world’s lightest solid material, consisting of 99.8% air. Despite weighing almost nothing, it can support its weight thousands of times.
NASA uses this transparent substance to capture space dust while architects explore its potential as super-insulation.
Like Go2Tutors’s content? Follow us on MSN.
Carbon Nanotubes’ Super Strength
These microscopic tubes of carbon are stronger than steel yet lighter than plastic. A single strand could theoretically lift an elevator into space.
Engineers currently use them in everything from tennis rackets to body armor.
Hydrophobic Materials’ Water Fear

These substances repel water so effectively that droplets bounce off like rubber balls. Inspired by lotus leaves, these materials are used in waterproof electronics and self-cleaning windows.
Some coatings can even make water roll uphill.
Superfluid Helium’s Climbing Act

When cooled near absolute zero, liquid helium loses all viscosity and can climb container walls. This quantum state allows it to flow without any friction, even against gravity.
Laboratory videos show it appearing to defy basic physics.
Like Go2Tutors’s content? Follow us on MSN.
Nitinol’s Memory Metal

This nickel-titanium alloy “remembers” its original shape when heated. Bend it, heat it, and watch it spring back to its programmed form.
Medical devices use this property for non-invasive surgeries, while architects employ it in earthquake-resistant buildings.
Graphene’s One-Atom Wonder
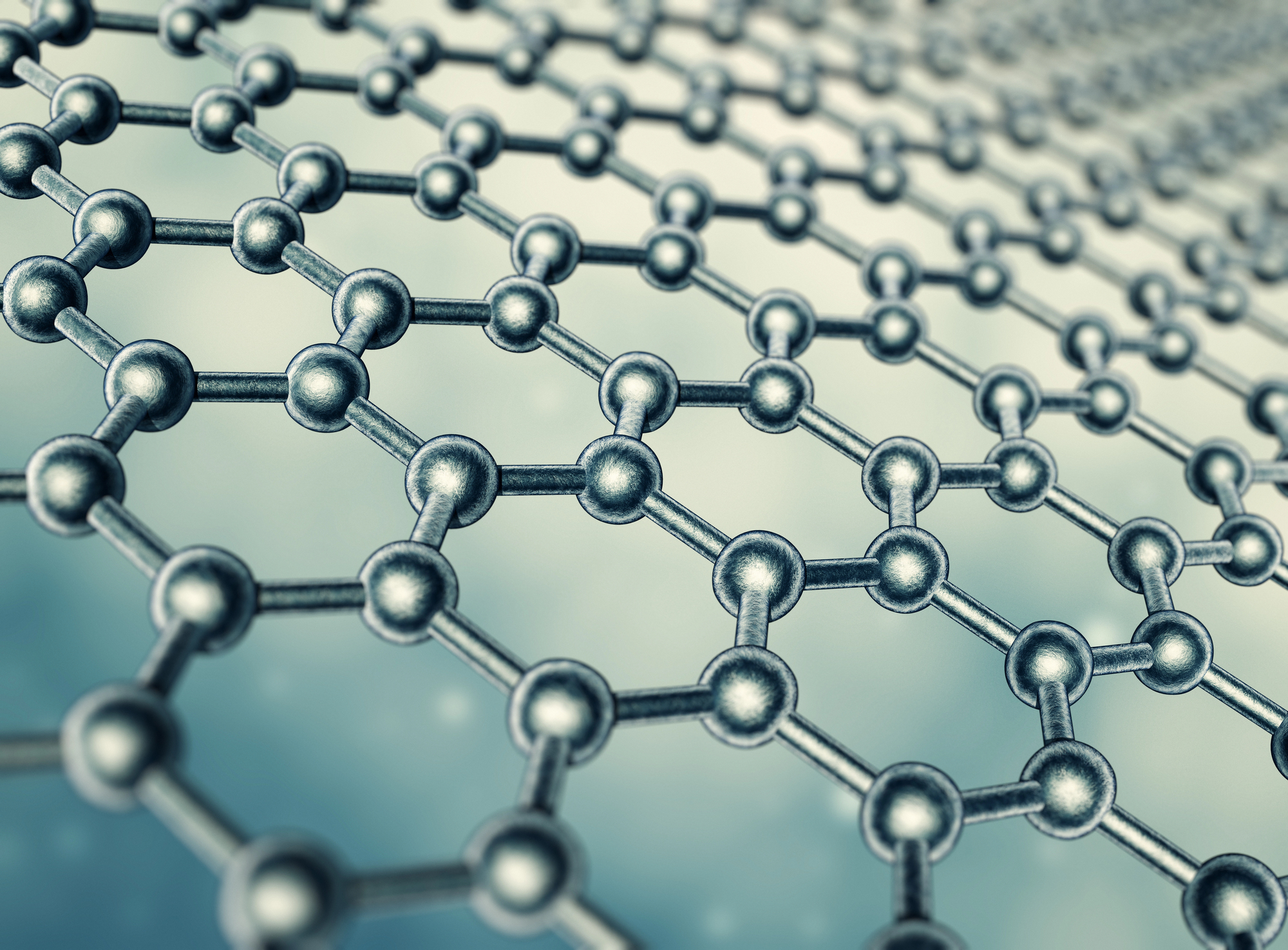
A single layer of carbon atoms creates the strongest, thinnest, and most conductive material known. Transparent yet impermeable to gases, graphene conducts electricity better than copper while being 200 times stronger than steel.
Amorphous Metals’ Liquid Structure

Unlike normal metals, these alloys lack a crystalline structure, making them incredibly strong and elastic. Golf club manufacturers use them for more powerful drives, while transformer makers value their magnetic properties.
Like Go2Tutors’s content? Follow us on MSN.
Piezoelectric Crystals’ Pressure Power

These crystals generate electricity when squeezed and change shape when electrified. Found in everything from cigarette lighters to guitar pickups, they convert mechanical stress into electrical charge and back again.
Quantum Dots’ Size-Shifting Color

These nanoscale semiconductors change color based solely on their size. The same material appears red when larger and blue when smaller, without changing its composition.
Television manufacturers use them for ultra-vivid displays.
Phase-Change Materials’ Temperature Control
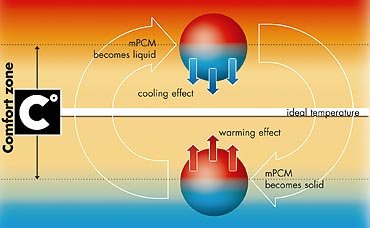
These substances absorb and release heat while maintaining a constant temperature. Originally developed for spacecraft, they now regulate temperature in building materials and clothing.
Some fabrics using them can keep wearers comfortable in both hot and cold conditions.Like
Go2Tutors’s content? Follow us on MSN.
Photochromic Compounds’ Light Response
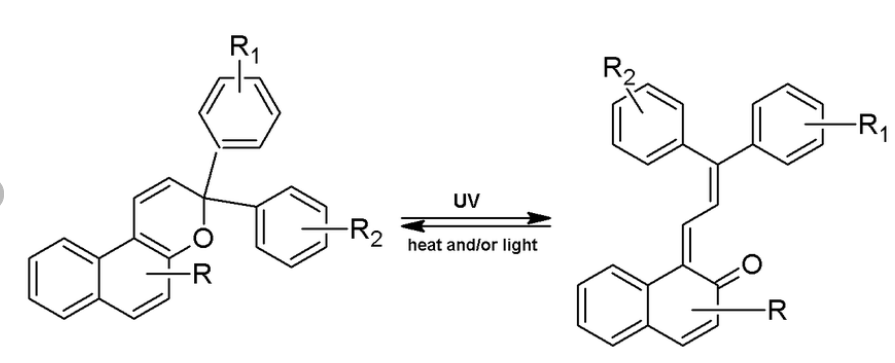
These molecules change color when exposed to light, as seen in self-tinting eyeglasses. The transformation occurs at the molecular level, rearranging atomic bonds in response to ultraviolet radiation.
Superconductors’ Perfect Flow

At extremely low temperatures, these materials conduct electricity with zero resistance. Magnetic resonance imaging machines rely on them for powerful magnetic fields while researchers explore their potential for loss-free power transmission.
Self-Healing Polymers’ Repair Magic

These plastics can repair their cracks and scratches. Some versions need heat or light, while others work at room temperature.
Mobile phone manufacturers are exploring their use of self-repairing screens.
Like Go2Tutors’s content? Follow us on MSN.
Liquid Crystals’ Organized Chaos
These substances flow like liquids while maintaining crystalline organization. Beyond their famous use in displays, they find applications in temperature sensors and stress analysis of structures.
Metamaterials’ Light Bending
These engineered materials interact with light in ways that natural materials cannot, potentially enabling invisibility cloaks. Their structure rather than composition creates their unusual properties, leading to applications in super-lenses and stealth technology.
Tungsten’s Extreme Resilience

This metal holds the record for the highest melting point of any pure element, remaining solid at temperatures that would vaporize most other materials. While used in light bulb filaments and rocket nozzles, tungsten withstands temperatures above 3,400°C (6,152°F).
Space agencies rely on its extraordinary heat resistance for spacecraft heat shields, while metallurgists study its unique crystal structure to understand extreme material properties.
Like Go2Tutors’s content? Follow us on MSN.
The Future of Chemical Innovation
These extraordinary substances demonstrate how chemical properties can transcend ordinary expectations. As research continues, new materials with even more remarkable properties emerge, promising innovations in technology, medicine, and engineering.
Each discovery expands our understanding of matter’s fundamental nature while opening new possibilities for practical applications.
More from Go2Tutors!

- 15 Unforgettable Candy Bars From The 60s and 70’s That Disappeared Too Soon
- 15 Myths About Famous Historical Figures That Aren’t True
- Famous Battles: How Much Do You Really Know About U.S. History?
- 20 Historical Artifacts That Scientists Can’t Explain
- 15 Little-Known Facts About Famous Historical Events
Like Go2Tutors’s content? Follow us on MSN.